Advanced use¶
Adjusting parameters¶
There are a number of important considerations when running inStrain. Here is some theory and data about how to make inStrain work best
Reference genome selection¶
inStrain relies on mapping reads from a sample to a reference genome. How similar the reference genome is to the reads, and the minimum read ANI threshold that you set, are very important and will determine much of what you get out of inStrain.
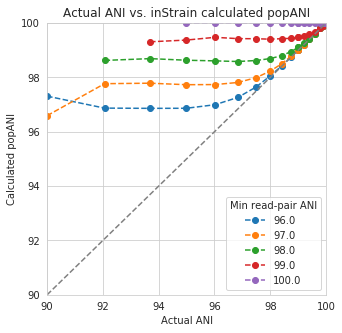
Below are a series of plots made by introducing a known number of mutations into an E. coli genome, simulating reads from these mutated genomes (at 20x coverage) with known ANI differences from the original reference genome, mapping the synthetic reads back to the original reference genome, and running inStrain.
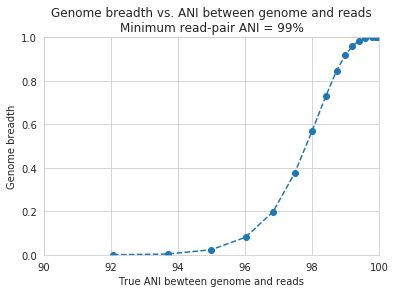
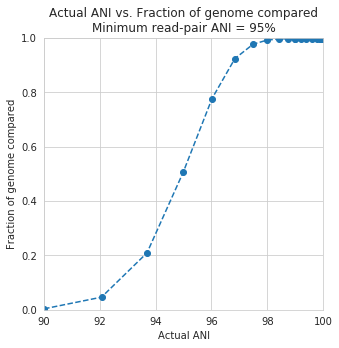
In the above plot, inStrain was run with a minimum read ANI of 0.99 (inStrain profile parameter -l or –min_read_ani). The reported genome breadth is reported on the y-axis. At 20x coverage, you should see 100% genome breadth (meaning that every base of the reference genome is covered by at least one read). However, when the reference genome is sufficiently different from the reads, the breadth is much lower. This is because when the read pair differs from the reference base by more than 99% ANI, it gets filtered out, and no longer maps to the genome. This can be exemplified a bit better by showing a variety of read filtering thresholds simultaneously:
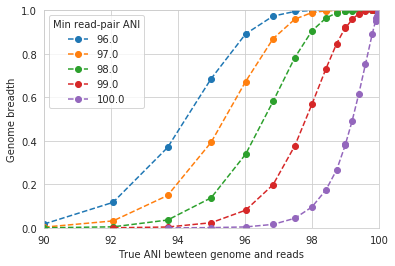
The line drawn in the first figure is now in red on this second figure. As you can see, the more you relax the minimum read ANI, the more you can align reads to more distantly related reference genomes.
Warning
You don’t want your minimum read pair ANI to be too relaxed, because then you risk mapping reads that don’t actually belong to the population represented by your reference genome (“non-specific” mapping). You can also avoid non-specific mapping by increasing the size of your reference genome dataset (more on that below)
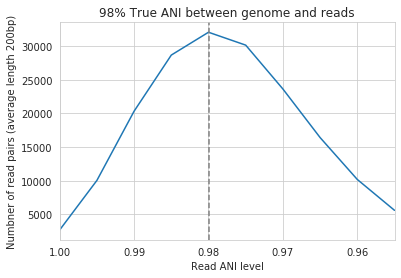
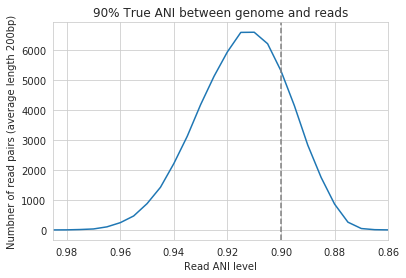
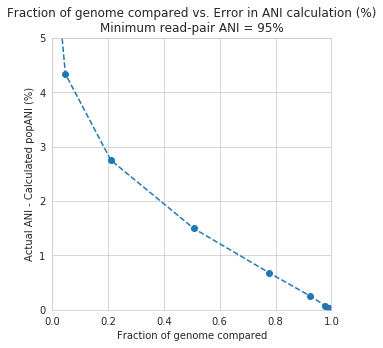
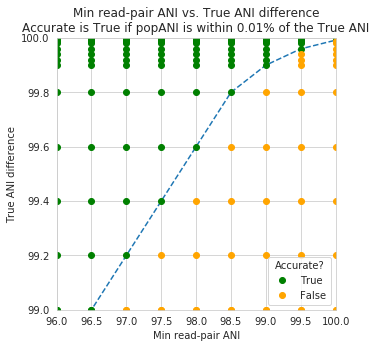
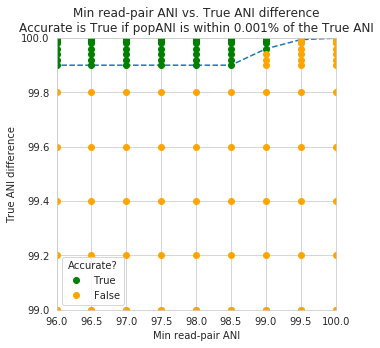
An important takeaway from the above figure is that the minimum read ANI should be at least 3% lower than the expected differences between your reads and the reference genome. If you look at the genome that’s 96% ANI from the reads, for example, you see that none of the minimum read ANI levels get the correct breadth of 1. If you look at the genome that’s 98% ANI from the reads, you can see that having a minimum read ANI of 96% is the only one that’s actually near 100% breadth. This can also be visualized by looking at the distribution of ANI values of read pairs mapping to the 98% genome:
Most read pairs have 98%, as expected, but there is a wide distribution of read ANI values. This is because SNPs are not evenly spread along the genome, a fact that is even more true when you consider that real genomes likely have even more heterogeneity in where SNPs occur than this synthetic example.
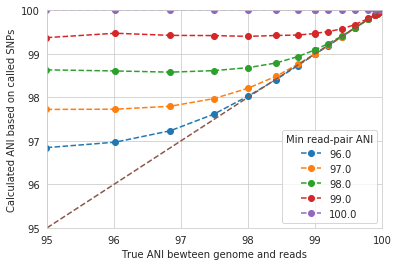
The fact that the reads fail to map to heterogenous areas of the genome is also more problematic than it originally seems. It means that the area of the genome that are most similar to the sample reads will recruit reads during read mapping, but the (potentially interesting) areas with more SNPs will not. This is exemplified in the figure below:
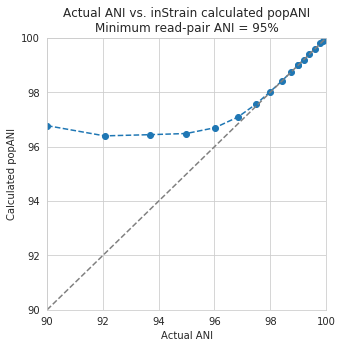
The y-axis in this figure shows the inStrain calculated ANI; that is, the number of identified SNPs divided by the number of bases with at least 5x coverage. If you look at red line, where only reads with at least 99% ANI are mapped, the ANI of reads mapping to the genome is almost always overestimated. This is because reads are only mapping to a small fraction of the genome (see the breadth in the second figure), and the small fraction of the genome that the reads are mapping to are the regions with a small number of SNPs.
By staring at this figure like I have, you’ll notice that the correct ANI is identified when the minimum read pair ANI is 2-3% lower than the actual difference between the reads and the genome. 96% minimum ANI reads correctly identify the ANI of the 98% genome, for example.
Finally, in case you’re wondering what the maximum read ANI is that bowtie2 is able to map, the answer is that it’s complicated:
When mapping to a genome that is 90% ANI to the reads, you no longer see a peak at 90% as you do in the 98% example. This is because bowtie2 doesn’t have a string ANI cutoff, it just maps what it can. This likely depends on where the SNPs are along the read, whether they’re in the seed sequence that bowtie2 uses, etc. While bowtie2 can map reads that are up to 86% ANI with the reference genome, I wouldn’t push it past 92% based on this graph.
Note
In conclusion, you want your reference genome to be as similar to your reads as possible, and to set your minimum read-pair ANI to at least ~3% lower than the expected different from the reads and the reference genome. The inStrain default is 95% minimum read pair ANI, which is probably ideal in the case that you’ve assembled your reference genome from the sample itself. If you plan on using inStrain to map reads to a genome that you downloaded from a reference database, you may want to lower the minimum read-pair ANI to as low as ~92%, and ensure that the genome your mapping to is at least the same species as the organism in your reads (as genomes of the same species share ~95% ANI)
Mapping to multiple reference genomes¶
Mapping to multiple genomes simultaneously to avoid mis-mapping¶
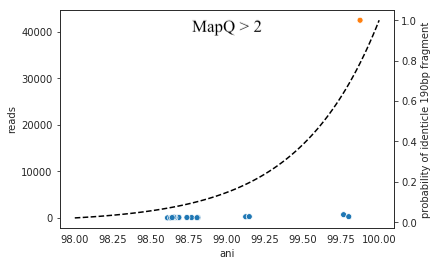
There are a number of ways to avoid mis-mapped reads (reads from a different population mapping to your reference genome). One method is to filter out distantly related reads, including by using the minimum read-pair ANI threshold (-l, –min_read_ani) or by using the mapQ score cutoff (more on that later). Another method is to include multiple reference genomes in the .fasta file that you map to, which gives the mapping software a chance to better place your reads.
When bowtie2 maps reads, by default, it only maps reads to a single location. That means that if a read maps at 98% ANI to one scaffold, and 99% ANI to another scaffold, it will place the read at the position with 99% ANI. If the read only maps to one scaffold at 98% ANI, however, bowtie2 will place the read there. Thus, by including more reference genome sequences when performing the mapping, reads will end up mapping more accurately overall.
Based on the above information, if you’d like to run inStrain on multiple reference genomes for the same set of reads, you should concatenate the genomes first and map to the concatenated genome set. You can then use inStrain genome_wide to get information on each genome individually.
Note
You can get an idea of the extent of mis-mapping going on in your sample by looking at the variation in coverage across the genome. If you see a region of the genome with much higher coverage than the rest, it is likely that that region is recruiting reads from another population. Looking at these wavy coverage patterns can be confusing, however. Here is a link for more information on this phenomenon.
Warning
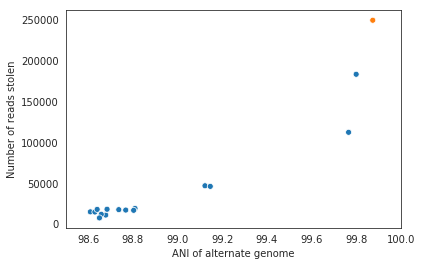
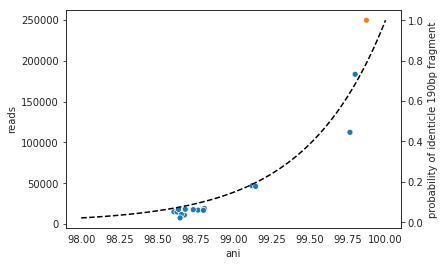
It is possible to include too many genomes in your reference .fasta file, however. You generally don’t want to have genomes that are over 98% ANI to each other in your reference genome set, because then the genomes can steal reads from each other. More on that below.
Other considerations¶
A final aspect to consider is de novo genome assembly. When multiple closely related genomes are present in a sample, the assembly algorithm can break and you can fail to recover genomes from either organism. A solution to this problem is to assemble and bin genomes from each metagenomic sample individually, and dereplicate the genome set at the end. For more information on this, see the publication “dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication”
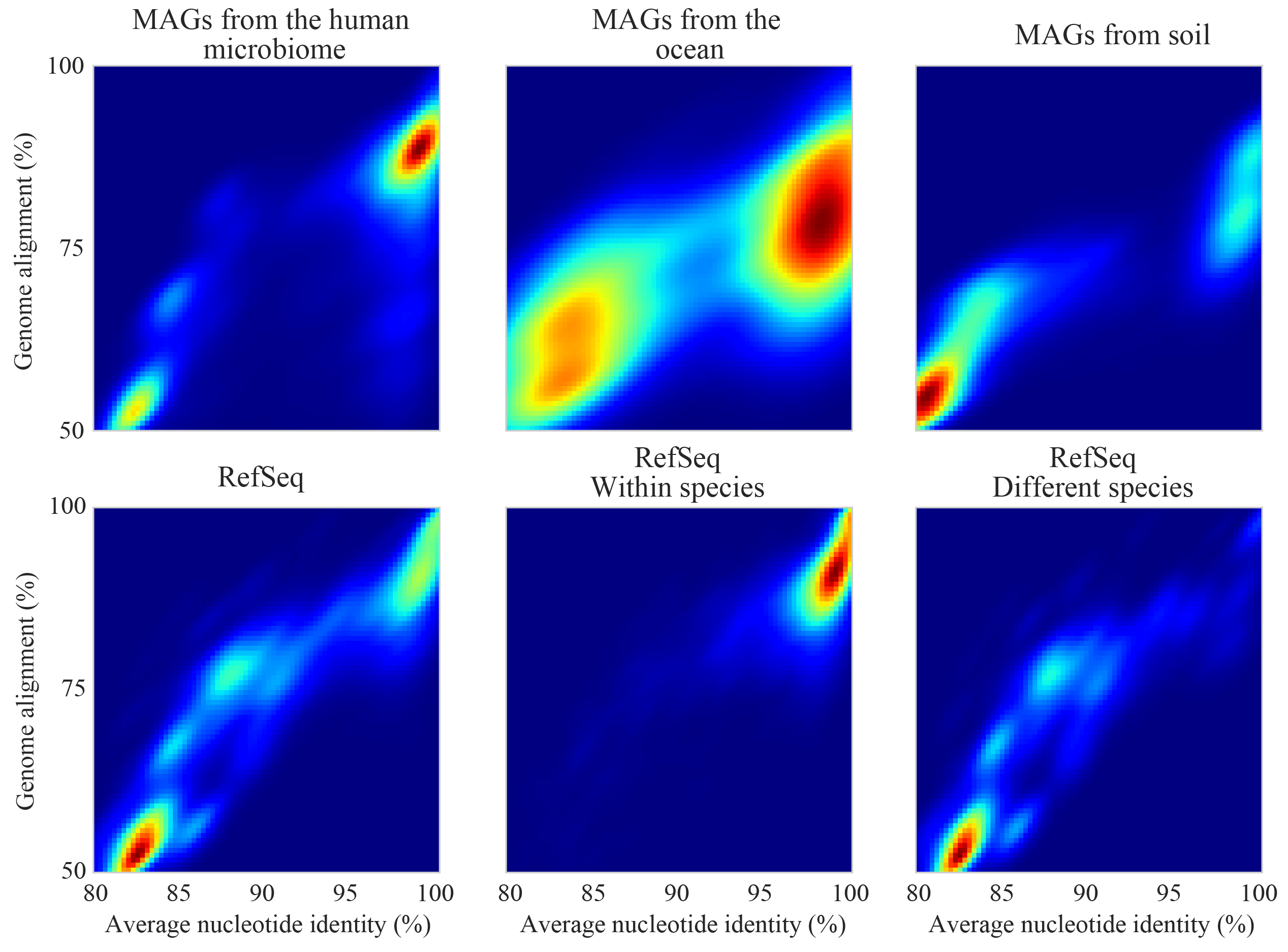
Assuming you de-replicate your genomes at 98% before mapping to run inStrain, another matter to consider is how you define detection of a genome in a sample. The following figure shows the expected genome overlap between genomes of various ANI values from different environments (adapted from “Consistent metagenome-derived metrics verify and define bacterial species boundaries”)
As you can see, genomes from that share >95% ANI tend to share ~75% of their genome content. Thus, using a breadth detection cutoff of somewhere around 50-75% seems to be reasonable.
Note
Based on the above information we recommend the following pipeline. 1) Assemble and bin genomes from all samples individually. 2) Dereplicate genomes based on 97-98% ANI. 3) Concatenate all dereplicated genomes into a single .fasta file, and map reads from all original samples to this concatenated .fasta file. 4) Use inStrain to profile the strain-level diversity of each microbial population (represented by a genome in your concatenated .fasta file)
Accessing raw data¶
inStrain stores much more data than is shown in the output folder. It is kept in the raw_data folder, and is mostly stored in compressed formats (see the section “Descriptions of raw data” for what kinds of data are available). This data can be easily accessed using python, as described below.
To access the data, you first make an SNVprofile object of the inStrain output profile, and then you access data from that object. For example, the following code accessed the raw SNP table
import inStrain
import inStain.SNVprofile
IS = inStain.SNVprofile.SNVprofile(``/home/mattolm/inStrainOutputTest/``)
raw_snps = IS.get('raw_snp_table')
You can use the example above (IS.get()) to access any of the raw data described in the following section. There are also another special things that are accessed in other ways, as described in the section “Accessing other data”
Basics of raw_data¶
A typical run of inStrain will yield a folder titled “raw_data”, with lots of individual files in it. The specifics of what files are in there depend on how inStrain was run, and whether or not additional commands were run as well (like profile_genes).
There will always be a file titled “attributes.tsv”. This describes some basic information about each item in the raw data. Here’s an example:
name value type description
location /Users/mattolm/Programs/strains_analysis/test/test_data/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS value Location of SNVprofile object
version 0.6.0 value Version of inStrain
bam_loc N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam value Location of .bam file
scaffold_list /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/scaffold_list.txt list 1d list of scaffolds, in same order as counts_table
counts_table /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/counts_table.npz numpy 1d numpy array of 2D counts tables for each scaffold
scaffold2length /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/scaffold2length.json dictionary Dictionary of scaffold 2 length
window_table /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/window_table.csv.gz pandas Windows profiled over (not sure if really used right now)
raw_linkage_table /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/raw_linkage_table.csv.gz pandas Raw table of linkage information
raw_snp_table /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/raw_snp_table.csv.gz pandas Contains raw SNP information on a mm level
cumulative_scaffold_table /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/cumulative_scaffold_table.csv.gz pandas Cumulative coverage on mm level. Formerly scaffoldTable.csv
cumulative_snv_table /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/cumulative_snv_table.csv.gz pandas Cumulative SNP on mm level. Formerly snpLocations.pickle
scaffold_2_mm_2_read_2_snvs /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/scaffold_2_mm_2_read_2_snvs.pickle pickle crazy nonsense needed for linkage
covT /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/covT.hd5 special Scaffold -> mm -> position based coverage
snpsCounted /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/snpsCounted.hd5 special Scaffold -> mm -> position based True/False on if a SNPs is there
clonT /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/clonT.hd5 special Scaffold -> mm -> position based clonality
mapping_info /home/mattolm/Bio_scripts/TestingHouse/N5_271_010G1_scaffold_min1000.fa-vs-N5_271_010G2.sorted.bam.v6.IS/raw_data/mapping_info.csv.gz pandas Report on reads
This is what the columns correspond to:
- name
- The name of the data. This is the name that you put into
IS.get()to have inStrain retrieve the data for you. See the section “Accessing raw data” for an example. - value
- This lists the path to where the data is located within the raw_data folder. If the type of data is a value, than this just lists the value
- type
- This describes how the data is stored. Value = the data is whatever is listed under value; list = a python list; numpy = a numpy array; dictionary = a python dictionary; pandas = a pandas dataframe; pickle = a piece of data that’s stored as a python pickle object; special = a piece of data that is stored in a special way that inStrain knows how to de-compress
- description
- A one-sentence description of what’s in the data.
Warning
Many of these pieces of raw data have the column “mm” in them, which means that things are calculated at every possible read mismatch level. This is often not what you want. See the section “Dealing with mm” for more information.
Accessing other data¶
In addition to the raw_data described above, there are a couple of other things that inStrain can make for you. You access these from methods that run on the IS object itself, instead of using the get method. For example:
import inStrain
import inStain.SNVprofile
IS = inStain.SNVprofile.SNVprofile(``/home/mattolm/inStrainOutputTest/``)
coverage_table = IS.get_raw_coverage_table()
The fellowing methods work like that:
- get_nonredundant_scaffold_table()
- Get a scaffold table with just one line per scaffold, not multiple mms
- get_nonredundant_linkage_table()
- Get a linkage table with just one line per scaffold, not multiple mms
- get_nonredundant_snv_table()
- Get a SNP table with just one line per scaffold, not multiple mms
- get_clonality_table()
- Get a raw clonality table, listing the clonality of each position. Pass nonredundant=False to keep multiple mms
Dealing with “mm”¶
Behind the scenes, inStrain actually calculates pretty much all metrics for every read pair mismatch level. That is, only including read pairs with 0 mis-match to the reference sequences, only including read pairs with >= 1 mis-match to the reference sequences, all the way up to the number of mismatches associated with the “PID” parameter.
For most of the output that inStrain makes in the output folder, it removes the “mm” column and just gives the results for the maximum number of mismatches. However, it’s often helpful to explore other mismatches levels, to see how parameters vary with more or less stringent mappings. Much of the data stored in “read_data” is on the mismatch level. Here’s an example of what the looks like (this is the cumulative_scaffold_table):
,scaffold,length,breadth,coverage,coverage_median,coverage_std,bases_w_0_coverage,mean_clonality,median_clonality,unmaskedBreadth,SNPs,breadth_expected,ANI,mm
0,N5_271_010G1_scaffold_102,1144,0.9353146853146853,5.106643356643357,5,2.932067325774674,74,1.0,1.0,0.6145104895104895,0,0.9889923642060382,1.0,0
1,N5_271_010G1_scaffold_102,1144,0.9353146853146853,6.421328671328672,6,4.005996333777764,74,0.9992001028104149,1.0,0.6748251748251748,0,0.9965522492489882,1.0,1
2,N5_271_010G1_scaffold_102,1144,0.9423076923076923,7.3627622377622375,7,4.2747074564903285,66,0.9993874800638958,1.0,0.7928321678321678,0,0.998498542620078,1.0,2
3,N5_271_010G1_scaffold_102,1144,0.9423076923076923,7.859265734265734,8,4.748789115369562,66,0.9992251555869703,1.0,0.7928321678321678,0,0.9990314705263914,1.0,3
4,N5_271_010G1_scaffold_102,1144,0.9423076923076923,8.017482517482517,8,4.952541407151938,66,0.9992251555869703,1.0,0.7928321678321678,0,0.9991577528529144,1.0,4
5,N5_271_010G1_scaffold_102,1144,0.9458041958041958,8.271853146853147,8,4.9911156795536105,62,0.9992512780077317,1.0,0.8024475524475524,0,0.9993271891539499,1.0,7
As you can see, the same scaffold is shown multiple times, and the last column is mm. At the row with mm = 0, you can see what the stats are when only considering reads that perfectly map to the reference sequence. As the mm goes higher, so do stats like coverage and breadth, as you now allow reads with more mismatches to count in the generation of these stats. In order to convert this files to what is provided in the output folder, the following code is run:
import inStrain
import inStain.SNVprofile
IS = inStain.SNVprofile.SNVprofile(``/home/mattolm/inStrainOutputTest/``)
scdb = IS.get('cumulative_scaffold_table')
ScaffDb = scdb.sort_values('mm')\
.drop_duplicates(subset=['scaffold'], keep='last')\
.sort_index().drop(columns=['mm'])
The last line looks complicated, but it’s very simple what is going on. First, you sort the database by mm, with the lowest mms at the top. Next, for each scaffold, you only keep the row with the lowest mm. That’s done using the drop_duplicates(subset=['scaffold'], keep='last') command. Finally, you re-sort the DataFrame to the original order, and remove the mm column. In the above example, this would mean that the only row that would survive would be where mm = 7, because that’s the bottom row for that scaffold.
You can of course subset to any level of mismatch by modifying the above code slightly. For example, to generate this table only using reads with <=5 mismatches, you could use the following code:
import inStrain
import inStain.SNVprofile
IS = inStain.SNVprofile.SNVprofile(``/home/mattolm/inStrainOutputTest/``)
scdb = IS.get('cumulative_scaffold_table')
scdb = scdb[scdb['mm'] <= 5]
ScaffDb = scdb.sort_values('mm')\
.drop_duplicates(subset=['scaffold'], keep='last')\
.sort_index().drop(columns=['mm'])
Warning
You usually do not want to subset these DataFrames using something like scdb = scdb[scdb['mm'] == 5]. That’s because if there are no reads that have 5 mismatches, as in the case above, you’ll end up with an empty DataFrame. By using the drop_duplicates technique described above you avoid this problem, because in the cases where you don’t have 5 mismatches, you just get the next-highest mm level (which is usually what you want)
Performance issues +————–
inStrain uses a lot of RAM. In the log file, it often reports how much RAM it’s using and how much system RAM is available. To reduce RAM usage, you can try the following things:
- Use the
--skip_mmflag. This won’t profile things on the mm level (see the above section), and will treat every read pair as perfectly mapped - Use
quick_profileto figure out which scaffolds actually have reads mapping to them, and only run inStrain on those
A quick and dirty estimate of resources required (as of version 1.2.12):
The required RAM (in Gb) is 0.4 times the length of the .fasta being mapped to (in Mbp). This is assuming the whole genome is covered by at least 1 read; portions of the .fasta file that have 0 reads mapping do not count.
The runtime (in minutes) is 13 times the number of read base pairs in the input .bam file (in Gbp).
A note for programmers¶
If you’d like to edit inStrain to add functionality for your data, don’t hesitate to reach out to the authors of this program for help. Additionally, please consider submitting a pull request on GitHub so that others can use your changes as well.